3,740,000,000,000,000,000,000
If every haemoglobin molecule could carry four molecules of oxygen then one gram of haemoglobin could carry 1.39 ml of oxygen:
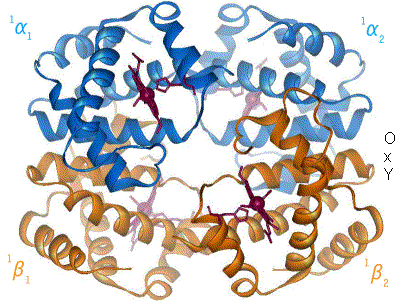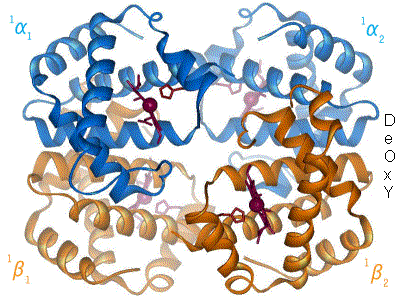
One molecule of haemoglobin will bind four molecules of oxygen
So, one 'mole' of haemoglobin will bind four moles of oxygen.
One mole of haemoglobin weighs 64,458.5 grams, and so one gram of haemoglobin is 1/64.458.5 of a mole of haemoglobin
1/64.458.5 mole of haemoglobin will bind four x 1/64,458.5 moles of oxygen = 6.21 x 10^-5 moles of oxygen
One mole of oxygen takes up 22.4 litres
6.21 x 10^-5 x 22.4 = 1.39 ml of oxygen
But, not all haemoglobin is functional - some is damaged (dyshaemoglobin), and some likely to be combined with carbon monoxide, and neither of these can carry oxygen. When the actual oxygen carrying capacity of haemoglobin is measured various values have been found, but 1.34 ml is often taken as the 'true' value, and is known as Hufner's number (or constant).
(BTW 1.39 ml of oxygen = 3,740,000,000,000,000,000,000 molecules of oxygen)
(Sorry, not really a picture! 😅)